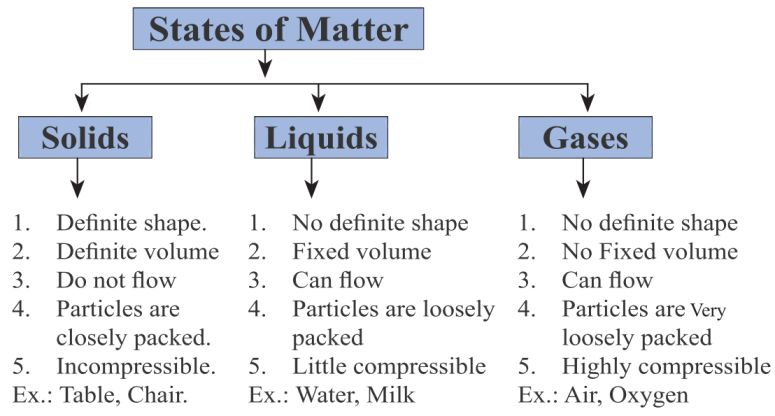
The different states of matter are:
- Solid – Has a definite shape and volume (e.g., ice, wood).
- Liquid – Has a definite volume but takes the shape of its container (e.g., water, oil).
- Gas – Has neither a definite shape nor volume, expanding to fill its container (e.g., oxygen, carbon dioxide).
- Plasma – A high-energy state where atoms lose electrons, found in stars and lightning (e.g., the Sun, neon signs).
- Bose-Einstein Condensate (BEC) – A state at extremely low temperatures where atoms behave as a single quantum entity.
There are also other exotic states, such as superfluid and quark-gluon plasma, but these are less common in everyday life.
Frequently asked questions
The main states of matter are solid, liquid, gas, and plasma. Additionally, Bose-Einstein condensate (BEC) is a state observed at extremely low temperatures.
Matter changes state through physical processes like melting, freezing, evaporation, condensation, and sublimation. These changes occur due to temperature and pressure variations.
Plasma is a high-energy state of matter where atoms lose electrons, creating charged particles. It is found in stars, lightning, and neon lights.
Bose-Einstein Condensate is an extremely low-temperature state where atoms slow down and behave as a single quantum entity. It was first created in a lab in 1995.
Yes, under certain conditions, a substance can exist in multiple states simultaneously. For example, at the triple point, water can exist as solid, liquid, and gas at the same time.
Yes, scientists have discovered exotic states of matter, such as quark-gluon plasma, which existed shortly after the Big Bang, and superfluid states, where matter flows without resistance. These states are studied in extreme conditions like high-energy physics experiments.
The key difference is that plasma consists of ionized particles (free electrons and positively charged ions), making it electrically conductive, while gas is neutral and does not conduct electricity.
Sublimation is the process where a solid directly changes into a gas without passing through the liquid state. An example is dry ice (solid CO₂), which turns into gas without melting.
Yes, apart from these four common states, scientists have discovered other states like Bose-Einstein Condensate (BEC), fermionic condensate, superfluid, and quark-gluon plasma, which occur under extreme conditions.