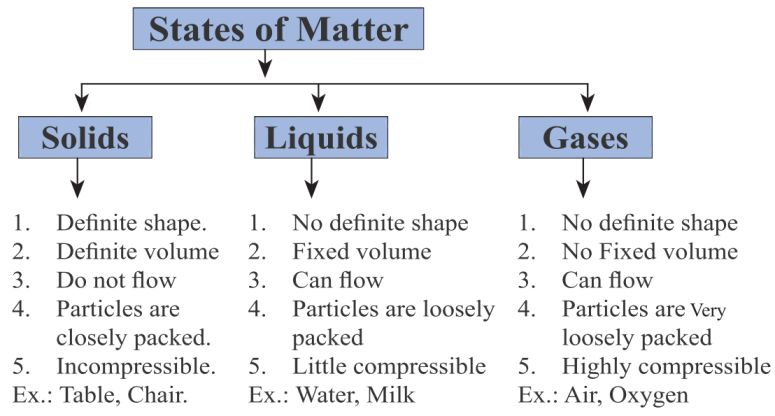
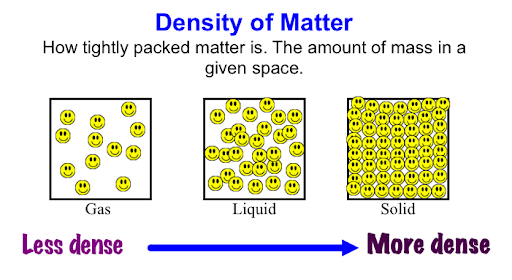
In general, solids have higher density than liquids because their particles are more tightly packed together. However, there are some exceptions.
For example:
- Water and Ice: Ice (solid) is less dense than liquid water, which is why ice floats on water.
- Metals and Mercury: Most metals are denser than mercury (a liquid), but mercury itself is denser than many other solids.
So, while solids usually have higher density than liquids, specific cases depend on the material and its molecular structure.
Frequently asked questions
→ Solids have tightly packed particles with strong intermolecular forces, making them denser than most liquids.
→ Ice has an open molecular structure due to hydrogen bonding, making it less dense than liquid water, so it floats.
→ Yes, mercury (a liquid metal) is denser than many solid metals like aluminum and iron.
→ Yes, increasing temperature usually lowers density by expanding the substance, while cooling increases density by contracting it.
→ Density is calculated using the formula: Density = Mass / Volume (ρ = m/V).