The components of a mixture can be separated using various methods including filtration, distillation, chromatography, evaporation, and magnetic separation, depending on the properties of the substances involved.
1. Filtration: This method separates a solid from a liquid using a filter that allows the liquid to pass through but traps the solid particles. Imagine making tea. You pour the hot water through a strainer (filter) to remove the loose tea leaves (solid) and obtain the tea (liquid).
2. Decantation: This is a simple method for separating two immiscible liquids (liquids that don’t mix) based on their density difference. Think of separating oil and water. Since oil is less dense, it floats on top of water. You can carefully pour off the top layer of oil (decantation) to separate it from the water.
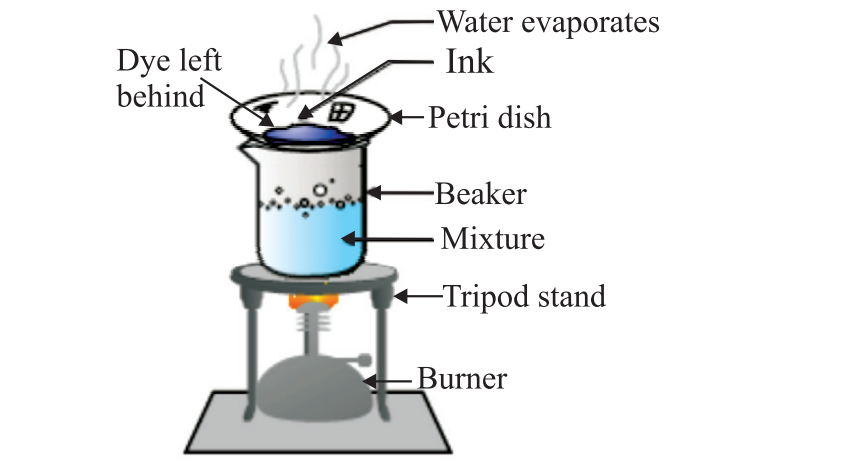
3. Evaporation: This method removes a liquid from a solution by heating it to the point where the liquid evaporates (turns into gas) and leaves the solid component behind. For instance, you can obtain salt from saltwater by heating the saltwater in a container. The water evaporates, leaving behind the salt crystals.
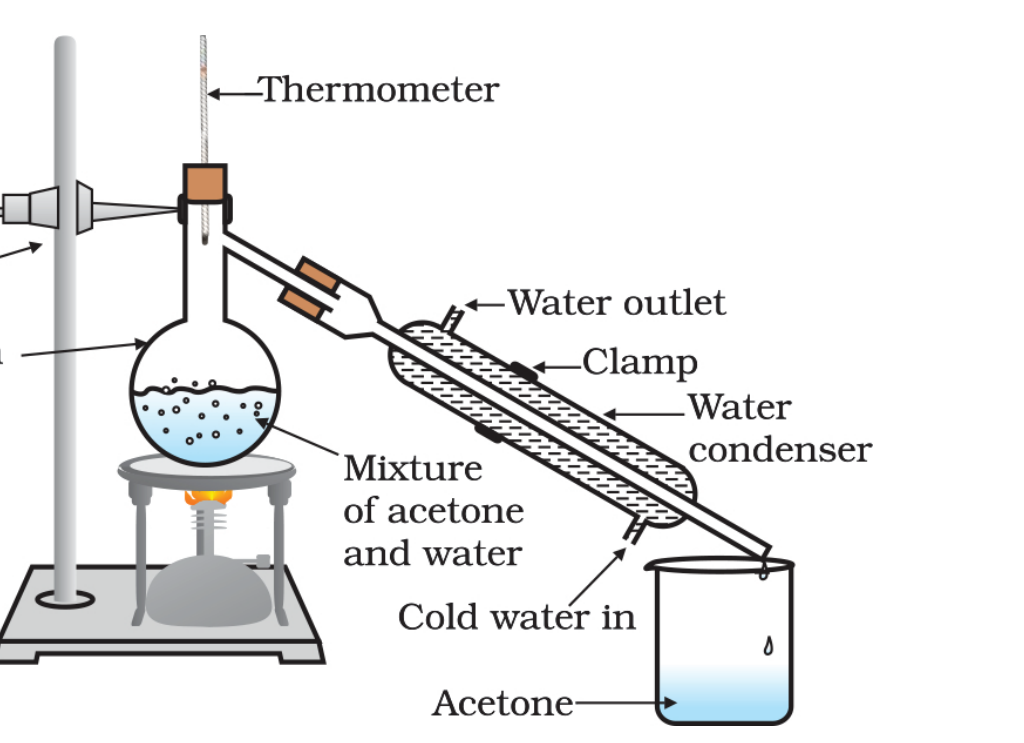
4. Distillation: This is a more advanced version of evaporation used to separate liquids with different boiling points. Imagine separating alcohol from a mixture of water and alcohol. The mixture is heated, and the alcohol with a lower boiling point vaporizes first. The vapor is then cooled and condensed back into liquid alcohol, leaving the water behind.
5. Crystallization: This technique purifies a solid dissolved in a liquid. The solution is concentrated by evaporation, and the desired solid crystallizes out as the solution becomes saturated. Making sugar crystals from sugar syrup is an example. As the water evaporates from the syrup, sugar reaches its saturation point and forms crystals.
6. Centrifugation: This method uses a centrifuge, a machine that spins a mixture at high speed. Centrifugation separates components based on their size and density. For example, separating cream (less dense) from milk (more dense) uses a centrifuge. The denser milk components settle at the bottom, while the cream floats on top.
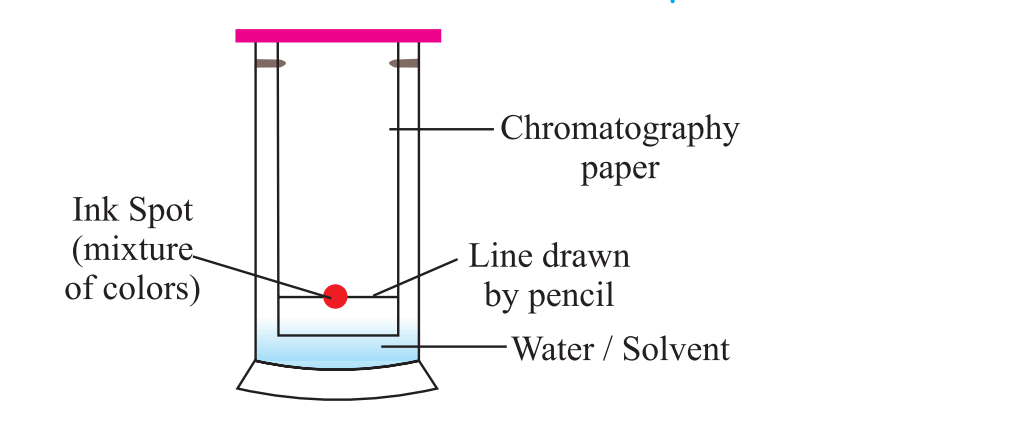
7. Chromatography: This more advanced technique is used to separate complex mixtures. It involves a stationary phase (like a special paper) and a mobile phase (like a solvent) that moves the mixture through the stationary phase. Different components in the mixture travel at different rates due to their varying interactions with the phases, allowing for separation. This technique is used to separate dyes, pigments, and other complex mixtures.