Answer: Kerosene and petrol are miscible liquids with a boiling point difference of more than 25ºC, they can be separated using simple distillation.
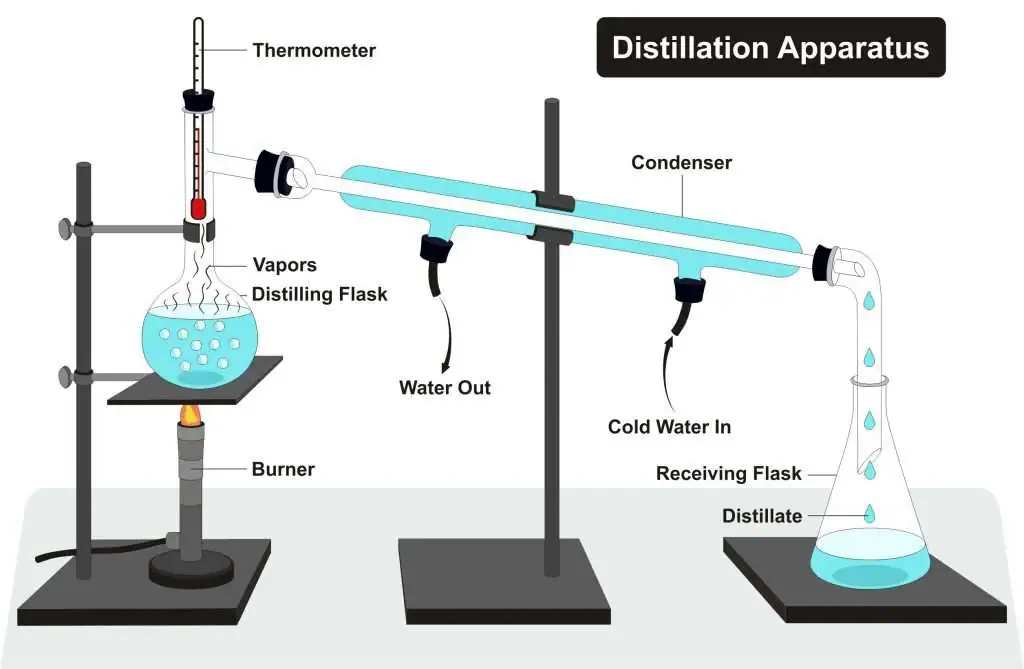
Steps:
- Heat the mixture in a distilling flask using a burner.
- Petrol evaporates first (lower boiling point) and forms vapors.
- Vapors pass through the condenser, where they cool down and turn into liquid.
- The distillate (petrol) is collected in the receiving flask.
- Kerosene remains in the distilling flask (higher boiling point).
Other Facts: Petroleum Refining Process
Thus, petrol and kerosene are successfully separated using this method
Petroleum is refined through fractional distillation and other processes to separate useful components.
Steps of Refining:
1. Separation (Fractional Distillation)
- Crude oil is heated in a fractionating column.
- Different fractions separate based on their boiling points:
- Gasoline (Petrol) – 40-200°C 🚗
- Kerosene – 150-250°C ✈️
- Diesel – 250-350°C 🚛
- Lubricating Oils & Residue – Above 350°C 🏭
2. Conversion (Cracking & Reforming)
- Cracking: Large hydrocarbons are broken into smaller, useful ones like petrol.
- Reforming: Improves fuel quality by rearranging molecules.
3. Purification (Treating & Blending)
- Sulfur removal to reduce pollution 🌱.
- Blending for better fuel performance.
Final Products:
✔️ Petrol (used in vehicles)
✔️ Diesel (trucks, trains)
✔️ LPG (cooking gas)
✔️ Kerosene (jet fuel)
✔️ Asphalt, Wax, Lubricants
Thus, petroleum is refined to produce valuable fuels and chemicals!

Related FAQs
🔹 The method used is simple distillation because the boiling point difference is more than 25°C, making it easy to separate the components.
🔹 The mixture is heated in a distillation flask.
🔹 Petrol (lower boiling point) evaporates first and is condensed in a separate container.
🔹 Kerosene (higher boiling point) remains in the flask and is collected separately.
🔹 Petrol: Around 40-200°C (varies due to different hydrocarbons).
🔹 Kerosene: Around 150-250°C.
🔹 Since the difference is more than 25°C, simple distillation is effective.
🔹 Fractional distillation is used when the boiling point difference is less than 25°C, requiring a fractionating column for efficient separation.
🔹 Since petrol and kerosene have a significant boiling point gap, simple distillation is sufficient.
🔹 Use proper ventilation as both kerosene and petrol are flammable.
🔹 Maintain a controlled heating rate to prevent overheating.
🔹 Avoid open flames to prevent fire hazards.
