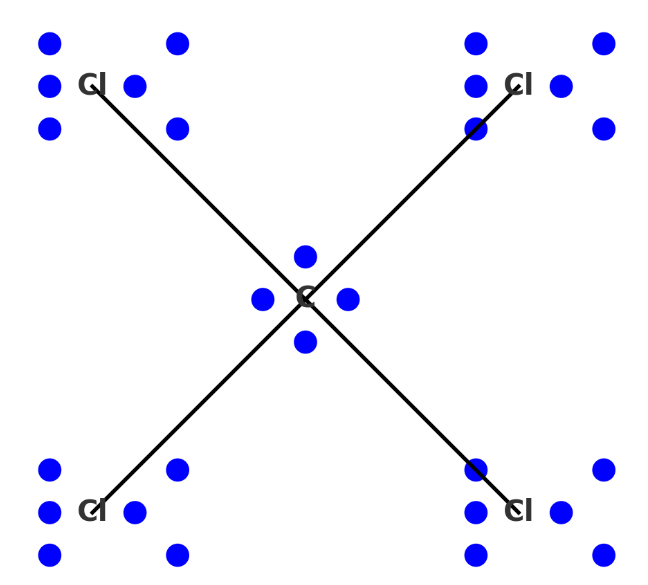
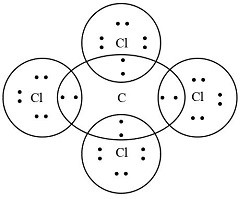
Electron dot structure (also known as the Lewis dot structure) for the carbon tetrachloride (CCl₄) molecule:
- The central carbon atom (C) is surrounded by four chlorine atoms (Cl).
- Each line between the carbon and a chlorine atom represents a covalent bond, where two electrons are shared.
- The dots around the chlorine atoms represent the electrons that are not involved in bonding (lone pairs).
This structure shows how carbon shares one electron with each chlorine atom, forming four covalent bonds to achieve a stable configuration.

