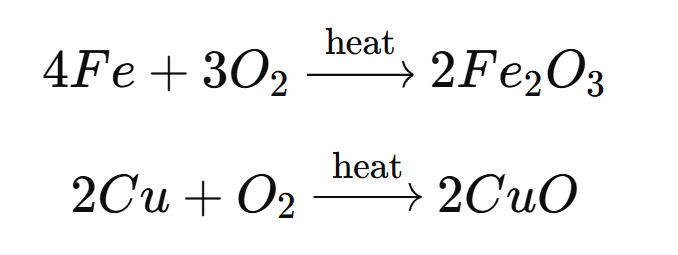Write the chemical equation for heating of Cu and Fe respectively
1. Heating of Copper (Cu) in the Presence of Oxygen: When copper is heated in the presence of oxygen, it forms copper(II) oxide. 2Cu + O₂ → 2CuO 2. Heating of Iron (Fe) in the Presence of Oxygen: When iron is heated in the presence of oxygen, it forms iron(III) oxide. 4Fe + 3O₂ → […]
Write the chemical equation for heating of Cu and Fe respectively Read More »