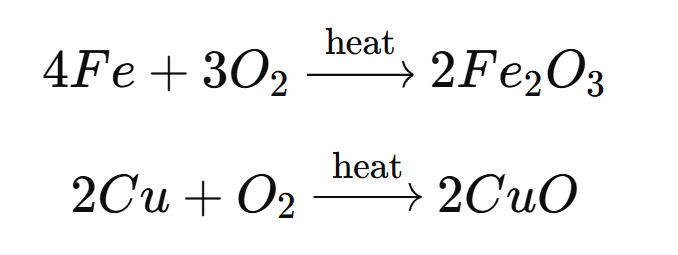Ionic compounds have high melting points because of the strong electrostatic forces of attraction between the oppositely charged ions in their structure. These forces, known as ionic bonds, hold the positive and negative ions together very tightly in a solid lattice structure.
To melt an ionic compound, a significant amount of energy is required to break these strong bonds and allow the ions to move freely. This is why ionic compounds, like sodium chloride (table salt), have much higher melting points compared to substances with weaker bonds, like covalent compounds.
Types of compounds
Compounds can be classified into several types based on the nature of the bonds between their atoms and their properties. Here are the main types:
► Ionic Compounds
- Bond Type: Ionic bonds (transfer of electrons between atoms).
- Examples: Sodium chloride (NaCl), magnesium oxide (MgO).
- Properties: High melting and boiling points, conduct electricity when dissolved in water or melted, usually solid at room temperature, and form crystalline structures.
► Covalent Compounds
- Bond Type: Covalent bonds (sharing of electrons between atoms).
- Examples: Water (H₂O), carbon dioxide (CO₂), methane (CH₄).
- Properties: Lower melting and boiling points compared to ionic compounds, do not conduct electricity, can be gases, liquids, or solids at room temperature.
► Metallic Compounds (Alloys)
- Bond Type: Metallic bonds (free electrons move throughout the metal lattice).
- Examples: Brass (copper and zinc), steel (iron and carbon).
- Properties: Good conductors of electricity and heat, malleable and ductile, high melting and boiling points, shiny appearance.
► Molecular Compounds
- Bond Type: Covalent bonds, but specifically refer to compounds formed by nonmetals.
- Examples: Glucose (C₆H₁₂O₆), ammonia (NH₃).
- Properties: Generally low melting and boiling points, can exist as gases, liquids, or solids at room temperature, do not conduct electricity.
► Complex Compounds
- Bond Type: Can involve ionic or covalent bonds, but feature a central metal atom bonded to a group of molecules or ions.
- Examples: Hemoglobin (iron complex in blood), chlorophyll (magnesium complex in plants).
- Properties: Have specialized functions, often found in biological systems.
► Acidic Compounds
- Bond Type: Often involve covalent bonds; they release hydrogen ions (H⁺) in solution.
- Examples: Hydrochloric acid (HCl), sulfuric acid (H₂SO₄).
- Properties: Sour taste, can conduct electricity in solution, react with metals and bases.
► Basic Compounds (Alkaline)
- Bond Type: Typically involve ionic bonds, often release hydroxide ions (OH⁻) in solution.
- Examples: Sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)₂).
- Properties: Bitter taste, slippery to the touch, conduct electricity in solution, react with acids.
► Organic Compounds
- Bond Type: Primarily covalent bonds, centered around carbon atoms.
- Examples: Methane (CH₄), ethanol (C₂H₅OH), proteins, lipids.
- Properties: Include a vast range of compounds, usually contain carbon-hydrogen bonds, found in all living organisms.
These categories help in understanding the different ways atoms combine to form compounds and the resulting properties these compounds exhibit.